Rickets
Old English term for Rickets was “Wrickken“ as described by Glisson F, Bate G and Regemorter A on 1651 as a common disease present in children. Near 350 years have been elapsed since the first monograph publication on wrickken and many spectacular advances in our understanding of Vitamin D dependent metabolism have enriched our knowledge wallet.
Last century was plenty of relevant discoveries from isolation of nuclear receptor for Vitamin D, identification of metabolic pathways of vitamin D synthesis, activation and degradation, until to isolation of genes coding for enzymes and co-factors as well’s hormonal factors involved into vitamin D cycle.
We can define rickets the defect in bone mineralization leading to formation of a normal bone matrix whithout deposition of calcium salts. Anatomically speaking this alteration in bone structure is called “osteomalacia”, whereas clinically propension to multiple fractures, short stature, bone deformities and renal alterations are often found. Initially the defect was ascribed to a loss of vitamin D expecially in young adults presenting lower exposure to sunlight; later it was clear that different forms of rickets are present; some involving abnormalities in vitamin D metabolism, other involving renal cells alterations.
It’s quite surprising that the horthologues of oncogenes designed such as “Interruptor-1” and “Interruptor-2” have been demonstrated to be involved into phosphate homeostasis. Int-1 is Wnt family growth factors, whereas Int-2 is FGFs family growth factor; both are very important in molecular embriology influencing cellular condensation and diffusion. Phosphate ions with carbon and azote ions, can be considered the base for live organisms in the earth, forming the backbone of all biomolecular structures. It is surprising that a so higly conserved and sophysticated biochemical pathway has been created to conserve adequate levels of phosphate in our organisms.
Interestingly, it is not at present clear the relation between bone loss and kidney stone formation. Excessive bone reabsorption clearly leads to hypercalciuria and hyperphsophaturia for example by excessive production of parathyroid hormone or vitamin D3. Excessive increase in urinary calcium excretion from bone can also be observed in osteogenesis imperfecta and in McCune-Albright syndrome; however, nephrolithiasis is very rare in these disorders.
On the other side, many studies have reported lower bone density in renal calcium stone formers compared to controls. The mechnisms underlying these findings is not clear, but may involve hyper responsiveness to calcitriol or to parathormone or bone abnormalities. However, low bone density in calcum stome formers has not been associated with increased bone resorption or with any gene mutations or polymorphisms.
According to my opinion, whereas osteoporotic bone disease can be truly considered an alteration of bone cells, osteomalacia (rickets) is better considered a bone symptom of a kidney disease. In the first situation we have altered bone turnover due to alterations of bone regulatory factors, in the second we have bone disease as secondary and sporadic manifestation of primary kidney disease.
Among the causes of defects of inadequate mineralization of bone (osteomalacia) and defective mineralization of cartilage (rickets) are renal phosphate wasting disorders that produce hypophosphatemia.
Kidney stones
Urinary PH determines the solubility of various substances in urine, Low PH decreases uric acid solubility but prevents calcium-phosphate crystal formation in contrast to a PH > 7 that augments urate solubility but precipitates calcium-phosphate salts. Urinary PH depends on the proton load in the diet and on the ability of kidney to buffer free proton in the urine. Renal acidosis is due to a defect of the renal tubule in secreting protons while buffers are normally produced, resulting in urinary PH > 5.5 and in metabolic acidosis. Patients with renal acidosis frequently have hypercalciuria and hyperphosphaturia. This may be due to calcium and phosphate release from bone because of proton buffering by bone.
Nephrolithiasis and nephrocalcinosis are frequent in these disorders. Distal renal acidosis is due to mutations in the chloride-bicarbonate exchanger or in proton ATP ase subunits.
Uric acid
Uric acid stones are less frequent than calcium stones and they represent 5 to 10% of nephrolithiasis. Uric acid is the final breakdown product of purine metabolism and it also derives from amino acid catabolism. Two-third of uric acid production are eliminated by the kidneys in humans. Uric acid is filtered at the glomerulus and then it is almost completely reabsorbed in the initial part of the proximal tubule by at least two transporters, called URAT1 , coded by gene SLC22A12) and GLUT9 ( coded by gene SLC2A9). A member of the ATP binding cassette family (ABCG2) is expressed in proximal tubular cells and secretes urate into the urine.
Two factors increase the risk of uric acid stone formation: low PH and hyperuricosuria. Genetic disorders can increase uric acid production or alter urate tubular transport. An increase in uric acid production induces hyperuricemia, as in the case of hypoxantine-guanine- phosphorybosyl transferase or in glucose-6-phosphatase deficiencies, and in phosporybosyl pyrophosphatase synthetase over-activity.
In contrast mutations in urate transporters result in hypouricemia and hyperuricosuria. Hence loss-of-function mutations in the URAT1 transporter decrease urate reabsorption in the proximal tubule. Functional experiments demonstrated that SLC9A2, is expressed at the apical and basolateral sides of proximal tubular cells, it transport urate, and that polymorphisms decrease urate reuptake increasing uric acid secretion in urine.
Inactivating mutations in tha ABCG2 gene have been identified as cause of gout increasing uric acid concentration in plasma.
Oxalate
Elevatd urinary oxalate excretion is critical for the growth of renal stones, Oxalate comes from the diet and it is produced by the liver and arythrocytes from glyoxalate. It is filtered freely at the glomerulus and probably reabsorbed ad then secreted in the proximal tubule. In the intestine, oxalate is also absorbed and secreted, but absorption exceeds secretion.
Enzymatic defects (primary hyperoxalurias) can induce oxalate overproduction.
The gene SLCA26A6 encodes an oxalate-chloride exchanger that is expressed in the intestine and in the renal proximal tubule. The disruption of this gene in mice results in an increase in oxalate plasma concentration, hyperoxaluria and renal calcium oxalate stone formation. The role of SLC26A6 is probably to secrete oxalate in feces. Its role in proximal tubule is not clear. Mutations in this gene have not been identified in humans.
Claudins
The selecive permeability of the intercellular unctions to calcium and magnesium ions is due to expression of claudin-16 , also known such as paracellin. This protein acts as a specific gate for clacium and magnesium. Mutations in claudin 16 abolished the permeability of the intercellular pathway to calcium, and are a rare cause of hypercalciuria, hypomagnesemia and calcium renal stones.
Variants of the gne enchoding claudin 14 have recently been asociated with kidney stones and low bone mineral density in a genome wide association study performed in patients from the Netherlands, Iceland, and Denmark, Claudin 14 is a tight junction protein expressed in the proximal tubule and in the loop of Henle. The mechanism by which these variants are associated with renal stones is unclear since they were not associated with calcium or phosphate concentration in plasma or urine, but only associted with serum PTH and bone markers.
OSTEOMALACIA
Vitamin D metabolism abnormalities
Rickets-Vitamin-D-dependent-type-I 1ahydroxylaseD3 (Ch12q14)
Rickets-Vitamin-D-dependent-type-II Vit.-D receptor (Ch12q13-14)
Rickets-PseudoVitamin-D-deficiency 1ahydroxylaseD3(Ch12q13.3) point mutation
Kidney Proximal tubular defect
Rickets hypophosphatemic with Hypercalciuria NTP2c gene (HHRH)(NPT2 genes Ch5q35.1-q35.3)
Autosomal dominant hypophosphatemic Rickets FGF23 gene (ADHR) OMIM 193100 of the young (Ch.12p13.3)
X Linked Hypophosphatemic Richets PHEX gene (HYP) like OMIM adult female (Ch. Xq22.1)
Tumor Induced Osteomalacia FRP4 gene(OHO) (Ch.7q14.1)
Hyperphosphatemic Familial Tumoral Calcinosis KLOTHO (HFTC) OMIM 21900
Hypeostosis-hyperphosphatemia syndrome GALNT3 (HHS) OMIM 610233
Renal Tubular Acidosis type II NPT2a gene (Fanconi’s Syndrome) (Ch5q35.1-q35.3)
Distal tubular defect
Renal-Tubular-Acidosis-type-I Basolateral- Cl/HCO3 (RTA Distal) Proton-ATPase
Hyperkalemic RTA type IV Hyporeninemic Hyperaldosteronism
Bartter’s syndrome Na-K-2Cl transporter mutation
(Henle loop hyperca) K channel-calcium sensing receptor
Chloride channel (CICKa-b)-Barttrin
Gitelman’s syndrome Na-Cl transporter thiazide sensitive
(distal hyperca)
Dent’s disease Voltage gated Chloride channel
INHERITED BONE DISEASES
Autosomal dominant Aplasia of FGF10 gene
Lacrimal and Salivary glands
Autosomal dominant Cerebellar FGF14 gene
Ataxia
Craniosynostosis disorders FGFR type 2 (Pro250X)
Achondroplasia FGFR type 3 (Ch4p16.3)
Hypochondroplasia FGFR type 3
Spondyloepiphyseal dysplasia * FGFR type 3 stop codon
Stickler syndrome * FGFR type 3 Lys650Glu
*thanatophoric dysplasia type I and type II.
Pfeiffer syndrome FGFR type 1 Pro252Arg
Apert syndrome FGFR type 2 Pro253Arg
Muenke craniosynostosis FGFR type 3 Pro 250Arg
Crouzon syndrome FGFR type 2 Cys342Arg
Jackson-Weiss syndrome FGFR type 2 Cys342Arg
Phosphate wasting is either inherited as X-linked hypophosphatemic rickets or autosomal dominant hypophosphatemic rickets, or acquired, as can occur in patients with a variety of benign mesenchimal tumors such as hemangiopericytomas, fibromas, angiosarcomas. Osteomalacia induced by tumors is invariably curable if the tumor can be found and resected, indicating that it may have an humoral basis. However the pathogenesis of rachitic syndromes requires also defective mineralization coupled with phosphate wasting.
Phosphatonins have been demonstrated to be able to impair the action of kidney 1a hydroxylasis, activating 24-hydroxylasis, and mediating the parathyroid action on many cell types, including kidney proximal cells.
However no clear action at bone forming units has been at present demonstrated by “phosphatonins” per se.
NPT2 and NHERF
We are understanding the mechanisms exerted by epithelial scaffold proteins in regulation of renal phosphate handling. These PDZ-containing proteins are able to form macromolecular complexes with true Na/Pi channels.
Called Na+/H+ Exchangers Regulatory Factor (NHERFs), they are known to be present on apical microvillar structure (NHERF1) or at the base of microvilli in the vescicle rich domains (NHERF2). They are ancillary cytoplasmic proteins, responsive to hormonal stimulation such as parathyroid hormone, and directing the localization of ion channel proteins (NPT2) at specific sites of cellular membrane. Their action has been demonstrated for microvillar apical membrane of proximal kidney tubular cells, but they probably, as PDZ-containing proteins, are ubiquitariously active in regulating the activity, internalization and recycling, of true receptors.
Their action is strictly dependent from transmembrane potential and finally from different concentration of sodium and potassium ions outside and inside cells respectively. As we know cell life is linked to the presence of this different concentration, allowing the formation of true concentration gradient across a lipid bilayer.
Moreover they are co-responsive of the presence of a difference in proton concentration (i.e.PH) across plasmamembrane. The PH difference across lipid bilayer between inner and extracellular fluid account for a different solubility or organic and inorganic salts as well’s of cathalization of enzymatic reactions possible only at a given PH.
Finally, these apparent unuseful PDZ-containing proteins should give the life to an inhert lipid bilayer; making it responsive to extracellular hormonal signals and so orchestrating the action of an uncohordinated lipid structure.
Their presence at specific sites of plasma membrane explaines the localization and activity of the products of SCL34A1 and SCL34A3 genes located on chromosome 5q35.1-35.3 and coding for (NPT2a) Na/Pi exchanger type IIa and (NPT2c) type IIc respectively. The sodium-phosphate cotransporter NTP-2c is responsible for the bulk of phosphate reabsorption in proximal renal tubules and its alteration is the cause of hypophosphatemic rickets with hypercalciuria. The putative “phosphatonin” should directly inhibit renal sodium-phosphate cotransporter.NPT2c is the primary hormone – regulated renal phosphate transporter, localized at the apical membrane of cells of proximal tubule in each nephron. It accounts for 80% of sodium dependent phosphate reabsorption.Interestingly dietary phosphate load causes a significant down-regulation of NPT-2c.
A distinct apical membrane sodium-phosphate cotransporter called NTP-2a is present in nephrons, sharing homologies with the former responsible for Proximal Renal Tubular Acidosis also called Fanconi’s Syndrome.It is quite consequential that the phosphaturic action exerted by FGF23 at kidney level is done by a down-regulation or block of the action of NTP-2 gene products mediating the reabsorption of phosphate ions.
Hyp mice experiments, showing a 50% reduction in NPT2, and an increase in FGF23 can be a perfect example of what happen in phosphate wasting syndromes.
FGF23
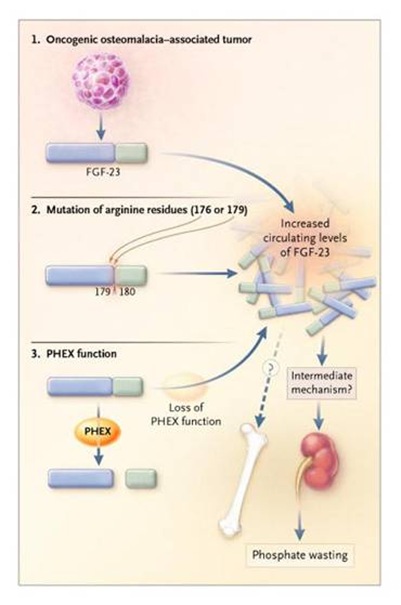
A paper of Shimada et al. on PNAS (2001) identifies a member of the fibroblast growth factor family, FGF23, as the humoral factor that is secreted by tumors to cause tumor-induced osteomalacia. They cloned a cDNA from a hemangiopericytoma that caused hypophosphatemic osteomalacia and found clones identical to FGF23, which was recently identified by positional cloning as the gene responsible for autosomal dominant hypophosphatemic rickets. When injected into mice the recombinant FGF23 produces mild phosphaturia and hypophosphatemia; interestingly Chinese Hamster Ovary (CHO) cells - FGF23, when grown as tumor in nude mice, fully reproduced the human syndrome of severe hypophosphatemia, growth retardation and rickets in the growth plates, deformities in the skeleton, reduced mineralized matrix and seems of unmineralized osteoid in bone. FGF 23 was expressed at high levels in the tumor from which it was cloned, and as recently reported by another group it is also expressed at high levels in other tumors associated with acquired osteomalacia.
Its expression in bone reach the highest level, in regions of active bone formation, a strong hybridization signal can be seen in osteoblasts lining bone surfaces. Newly formed osteocytes and osteoprogenitor cells are also labeled. In other tissues it has been detected in particular in parathyroid, thymus, brain, heart, and vascular system. If in the past some contraddictory results have been obtained it was due to difficulties in metods of measurements. In particular it is necessary to evaluate if we have to measure the entire FGF23 or only its biologically active portion, its C terminal (Ct) part.
With a 72 aa Ct domain not shared with other family members FGF23 is the largest member of the FGF family. Insight into its functions are provided by demostration that mutations caused hypophosphatemic rickets. Moreover it may seems more soluble that other family members lacking the heparing-binding motif presented in other FGFs.
Whyte KE demonstrated that four unrelated families had a missense mutations in one or two closely spaced arginine residues at position 176 and 179 that cosegregates with rickets, with two families sharing the same mutation. This clustering of missense mutations is a disorder with a dominant inheritance and strongly suggest a “gain of function mutation”.
Shimada et al demonstrated that arginine 179 and S180 is a processing site in FGF23, because they found in CHO cells expressing FGF23, in addition to the mature protein a fragmented protein beginning with S180. It is believed that mutations of the flanking arginine at position 179 could confer a gain of function on FGF23 by blocking its degradation at cleavage site between the unique Ct domain and the FGF-homologue regions.
FGF23 may be the long-sought “phosphatonin”, the phosphaturic factor normally accounting for phosphate homeostasis, independently from parathyroid hormone, so independently from calcium levels. It has been postulated that the main help to FGF23 secretion is the product between the concentration of Calcium and Phosphorus. So acquiring a relevance in regulation of ectopic calcifications such as those present in vascular system with aging.It may be that FGF23 is also secreted by one or more normal tissues as a phosphate regulating hormone, and that the blood level of FGF23 in blood is determined in part by the rate of cleavage by Subtilisin-like proprotein convertase (SPC) at R179/S180. Two fragments are present an entire protein of 32 kDa and C terminal segment of 12 kDa.On 2004 it was demonstrated the complete action of FGF23 on NTP2 renal cotransporter, giving the final answer to identification of FGF23 such as phosphatonin.
Quite recently a longitudinal study demonstrated that in dyalitic patients measurements of FGF23 plasma levels can be useful in assessing normo-phosphatemic patients who should benefits of therapeutic strategies devoted to manage phosphorus balance, considering that hyperphosphatemic hemodyalitic patients show an increased risk of death. This study suggests that hyperphosphatemia in these patients is only partially assessment of risk associated with abnormal phosphorus metabolism. However, measurement of FGF23 could represent a new biomarker in assessing the risk of death in patients with early kidney disease (Wolf M. NEJM August 7, 2008).
PHEX
The other piece of the hypophosphatemic puzzle is the X-linked hypophosphatemic rickets, the most common disorder of renal phosphate transporter.
PHEX belongs to the M13 family of MA clan of Zn-metalloendopeptidases. The prototypic member of this group of type of integral membrane glycoproteins is Neutral Endopeptidase (NEP). These proteins have a short cytoplasmic N-terminal region, a single transmembrane domain, and a large extracellular C-terminal domain with a zinc binding motif.
Other members of this group include:
. Endothelin Converting Enzyme-1 (ECE-1alfa, ECE-1beta and ECE-2)
. ECE-like enzyme/distress induced neuronal endopeptidase (ECEL1/DINE)
. Soluble Endopeptidase/NEP-like enzyme-1/Neprilysin-2 (NL1/NEP2)
. Membrane Matallo Endopeptidase-like 2 (MMEL-2)
. Kell Blood Group Protein antigen (KELL)
Neprilysin is aslo calle common acute lymphoblastic leukemia antigen (CALLA), or CD10, NEP, or Enkephilinase.
The M13 zinc metallo endopeptidases are integrally involved in several essential elements of cellular regulation and physiology as well’s in diseases including renal function defects, bone mineral loss disorder, cardiovascular diseases, arthritis nd inflammatory disorders.In particular PHEX gene is similar to those of NEP family in several important aspects: numebrs of small exons (22 exons characterized for PHEX with a sequence of 749 aminoacids), higly conserved aminoacid Zinc binding motif (HEFTH fof PHEX, HEITH for NEP).
Informations concerning the structure and nature of the PHEX gene product catalytic site was acquired from the analysis of 99 families affected by X Linked Hypophosphatemic Richets (HYP) and compter generated physichemical data and site-directed mutagenesis studies published for M13 and M3 metallo peptidases.
Interestingly full lenght FGF23 and MEFE do not appear to be PHEX substrates. Remarkably PHEX protects full-leght MEPE from proteolysis, notably by catepsin B cleavage in vitro. In addition, osteocalcin is not degraded by PHEX and inhibits PHEX cleavage of PTHrP.
PTHrP is one of the very few naturally occurring substrates cleaved by PHEX.
Osteocalcin is not cleaved by PHEX, the negatively charged Gla residues in osteocalcin are thought to interact with higly conserved charged present in PHEX. Similar charged region is present in MEPE and ASARM proteins.
In the intact MEPE and PHEX may be associated through the interaction with the MEPE C-terminal ASARM motif. This interaction may not necessarily lead to proteolysis. MEPE-PHEX interaction may therefore prevent proteolytic cleavage and release of ASARM peptide by protecting MEPE from localized matrix proteases. PHEX is localized on plasma membrane surface of osteoblasts, with its extracellular long C terminal region ideally situated in extracellular matrix for protein-protein interactions.
Several PHEX mutations has been detected in patients affected by X Linked Hypophosphatemic Richets (HYP) results in sequestration of disease causing PHEX in endoplasmic reticulum and subsequent failure to targeting to plasmamembrane.
PHEX play a major role in mineralization and it is expressed predominantly in bones and teeth.
The bone expression is localized into osteoblasts, osteocytes (not pre-osteoblasts); in teeth it is present in odontoblasts.
Interestingly loss of function of PHEX results in a defective mineralization. Its action on kidney is expressed modulating renal phosphate handling but not directly, suggesting a secondary involvement in regulation of a circulating systemic factor.
Finally it is reasonable to speculate that PHEX may well function as a small peptide protease and also as a matix-protein ligand.
SPC Subtilisin-like proprotein convertase
The SPC are a family of serine proteases, involved in processing of a wide variety of polypeptides including neuropeptides, growth factors, receptors, blood coagulation factors. Their substrates are cleaved at C terminal side where a specific sequence is present . SPC are present at Golgi apparatus and at trans Golgi network, where they act also on FGF23.
Matrix Extracellular phosphoglycoprotein (MEPE)
MEPE was first cloned from a tumor reseacted from a patients with OHO. It belong to a family of proteins that ahve recently been named Shorth Integrin Binding Ligand Interacting Glycoprotein (SIBLINGs). Between them we have:
. Osteopontin
. Matrix extracellular phosphoprotein (MEPE)
. Dentin Matrix Protein 1
. Bone Sialoprotein
. Dentin Sialo Phosphoprotein
. Enamelin
All mapping on chromosome 4q21 and sharing many properties. All these proteins have a links with bone/dentin mineralization and phosphate/calcium salts.
The structure of these proteins contains RGD motif tipical of integrin ligands, glycosylation pattern very similar between them, phosphorylation pattern again similar, and a so called ASARM motif.
ASRM (Acidic Serine Aspartate Rich MEPE associated motif) described a region of these protein able to block the mineralization. However if it is bounded to other extracellular matrix components it may be required a a nucleator of mineralization itself. The ASARM peptide is very stable and it is resistant to know proteases. Free ASARM peptides may also contribute to inhibition of renal phosphate uptake. This mechanism of action is likely to be steric and exacerbating the effect of NTP2 exchanger protein probably with the aids of FGF23.
The normal action of MEPE is to act such as mineralization inhibitor, due to the presence of ASARM fragment normally released from the entire protein by the cathepsin C cleavage.
Another action of MEPE is a dose dependent inhibition of BMP-2 menediated mineralization in a murine osteoblast cell line in vitro, another effect linked to ASARM presence.
Mice models
From studies in hypophosphatemic mice called “hyp mice” and “gyro mice” it was identified the cause of reduced phosphate reabsorption in a gene located in mouse chromosome X coding for PHEX a metalloproteinase enzyme. This proteins was defective in these mice; in hyp mouse the defect was localized primary in kidney, whereas in gyro mouse the defect was located also in the inner ear and so clinically associated with circling behaviour.
KO mice for Na-Pi cotransporter gene called Npt2, showed Pi renal wasting comparable to inactivation of PHEX gene, but in Npt2 KO mice calcitriol responds appropriately to hypophosphatemic challenges, intestinal absorption of both Pi and Ca ensues, and rickets and osteomalcia are absent.
MEPE KO mice have increased bone mass, resistance to aging associated trabecular bone loss, increased mineralization apposition rate and a dramatically accelerated mineralization rate in ex vivo osteoblasts cultures.
Interestingly Vitamin D3 Receptor KO mice have markedly increased levels of mRNA for MEPE expression.
Comparison between these mice models illustrates that renal phosphate wasting can be dissocated from defective synthesis of calcitriol, implying that phosphatonins have at least two independent actions:
1. they inhibit the Pi reabsorption
2. they impair the synthesis of calcitriol
The different phenotypes in Npt2 KO mice and PHEX KO mice also raise the possibility that FGF23 has a direct affect on bone and cartilage that contribute, along with hypophasphatemia to a defect in mineralization.
HYP mice model hepl us in understanding a possible explanation; the HYP mouse model of PHEX inactivation responds to phosphate deprivation, with continued phosphaturia relative to wilde type mice.
Normally phosphate can be cleared from urine by low dietary Pi intake, protecting against Pi depletion. So that decreased FGF23 secretion could be in this view the humoral factor of this response, coupling with at yet unidentifed phosphate sensor, possibly in the intestinal mucosa, to regulate intestinal phosphate reabsorption. Moreover FGF23 may explaine only a local paracrine regulatory role perhaps even unrelated to Pi homeostasis, and only when it is inappropriately secreted into blood may exert a Pi wasting action.
In this scenario, the phosphate wasting in tumor induced osteomalacia would be analogous to the Pi wasting that occurs when tumors overexpress the PTH-related peptides, PTH-rP. PTH-rP is normally a local regulator of cell differentiation, but when overproduction gives it access to the circulation, it co-opts the PTH receptor in kidney to cause phosphaturia.
Conerning FGF23 it is the first FGFs for which mutations are associated with a disease, and althought the othr 22 FGFs share only 4 known receptors, it is likely that FGF23 has a different receptor, because cleavage of its unique C terminal domain inactivates it.
Tumor Induced Osteomalacia
In literature about 70 cases have been described with such rare form of hypophosphaturia, which occurs in association with coexisting tumor and resolve after its excision; with a possible relapsing episodes.
Hypophosphatemia is probably due to a diminished renal phosphate reabsorption and this phenomenon causes a decrease in 1a hydroxylation of vitamin D3.
Tumor are generally of mesenchymal origin ( such as hemangiopericytomas), but also prostate and breast cancer have been described. They are often small and difficult to locate. From Cai Q data on 1994 it was described a “unidentified soluble factor” heat labile that is devoted normally to control renal phosphate reabsorption.
Oncogenic osteomalacia has been linked to secretion of a Frizzled receptor protein (FRP4) containing cysteine rich ligand binding domain as well’s hydrophilic C terminal region. The normally bound receptor link Wnt proteins in tandem with LPR family co-receptors. The binding to Wnt proteins to frizzled receptors and LPR5/6 coreceptors in heterotrimeric complexes on the cell surfaces leads to stabilization of intrcellular catenin beta and a complex network of singalling cascade.
It has been demonstrated in many cancers both in vivo and in vitro, that this pathways account for bone osteolysis in cancer diffusion to bone tissues.
Moreover some rare inherited disorders are characterized by involvement of Wnt/LPR pathways alterations:
. osteoporosis pseudoglioma syndrome : congenital blindness and severe chilhood osteoporosis
. High Bone Mass syndrome: associated with phenotypical presence of inherited high bone mass level.
FRP4 is located on chromosome 7p14.1 and it is conprised of six encoding exons, spanning 10.8 kb of genomic sequence. The translated protein product consist of 346 aminoacids, of which the first 21 residues constitute the predicted signal peptide. Finally the molecular mass of FRP4 is approximately of 40 kDa, but it is glycosylated to form a mature peptide of 48 kDa. It is ubiquitariously expressed, but importantly on bone cells it is present indicating a possible auto and paracrine effect in the skeleton too.
Concerning phosphate metabolism the study of Berndt et al (2003) revealed that this peptide has the capacity of inhibit the sodium dependent phosphate reuptake in opossum kidney in vitro experiments. Moreover infusion of FRP4 in vivo in parathyroidectomized mice caused an increase in the fractional excretion of phosphate and subsequent hypophosphatemia, indicating a mechansim of action partially independent from PTH.
Hyperphosphatemic Familial Tumoral Calcinosis
Recent work regarding fibroblast growth factor 23 addressed the question of pathogenesis of familial tumoral calcinosis. A missense mutation in the gene encoding FGF23 cause autosomal dominant hypophophatemic rickets. In addition FGF23 is highly expressed by tumors causing oncogenic hypophophatemic osteomalacia. FGF23 is therefore a strong candidate for “phophatonin”, the factor implicated as a cause of the phophate wasting in patients with oncogenic hypophosphatemic osteomalacia. The mutations tht cause autosomal dominant hypophosphatemic rickets stabilizes FGF23, potentially elevating its concentration in serum and leading to renal phosphate wasting. The same would be true in oncogenic hypophosphatemic osteomalacia: i.e. a great production of FGF23 by tumor and so increased levels of plasma FGF.
The clinical response to octreotide therapy as described by Seufert J in a patients may suggest that secretion of fibroblast growth factor 23 by the tumor can be modulated throught the somatostatin receptor signaling pathway. This protein (FGF23) has been demonstrated to be a substrate for the endopeptidase PHEX and to inhibit the phosphate transport in kidney cells. Moreover in autosomal dominant form of hypophosphatemic rickets, mutations in FGF23 have been identified that render the molecule resistant to cleavage by PHEX. So that in these patients octreotide scanning may be useful in identifying such a small tumors or relapses.
References
Resnick M, Pridgen DB, Goodman HO. Genetic predisposition to formation of calcium oxalate renal calculi. N Engl J Med 1968;278:1313-8.
Tieder M, Arie R, Modai D et al. Elevated serum 1,25-dihydroxyvitamin D3 concentration in siblings with primary Fanconi’s syndrome. N Engl J Med 1988;319:845-9.
Cai Q, Hodgsan SF, Kao PC et al. Brief report:inhibition of renal phosphate transport by a tumor product in a patient with oncogenic osteomalacia. N Engl J Med 1994;330:1645-9.
Econs MJ, Drezner MK. Tumor induced osteomalacia – unveiling a new hormone. N Engl J Med 1994;330:1679-81.
Seufert J, Ebert K, Muller J et al. Octreotide therapy for tumor induced osteomalacia. N Engl J Med 2001;345:1883-8.
Prié D, Huart V, Bakouh N et al. Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med 2002;347:983-91.
Kronenberg HM. NPT2a – The key to phosphate homeostasis. N Engl J Med 2002;347:1022-4.
Jonsson KB, Zahradnik R, Larsson T et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 2003;348:1656-63.
Carpenter TO. Oncogenic osteomalacia – A complex dance of factors. N Engl J Med 2003;348:1705-8.
Hesse E, Rosenthal H, Bastian L. Radiofrequency ablation of a tumor causing oncogenic osteomalacia. N Engl J Med 2007;357:422-4.
Karim Z, Gérard B, Bakouh N et al. NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med 2008;359:1128-35.
Gutiérrez OM, Mannstadt M, Isakova T et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008;359:584-92.
Reilly BM, Hart PD, Mascarell S et al. A question well put. N Engl J Med 2009;360:1446-51.
Magen D, Berger L, Coady MJ et al. A loss of function mutation in NaPiIIa and renal Fanconi’s syndrome. N Engl J Med 2010;362:1102-9.
Thorleifsson G, Holm H, Edvardsson V et al. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet 2009;41:926-30.
NPT2 cotransporter
Tenenhouse HS, Beck L. Renal Na+-phosphate cotrasporter gene expression in X linked Hyp and Gyro mice. Kidney Int 1996;49:1027-32.
Beck L, Karaplis AC, Amizuka N et al. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria and skeltal abnormalilites. Proc Natl Acad Sci USA 1998;95:5372-7.
Tenenhouse HS, Martel J, Gautier C et al. Renal expression of the sodium/phosphate cotransporter gene, Npt2, is not required for regulation of renal 1 alpha-hydroxylase by phosphate. Endocrinology 2001;142:1124-9.
White KE, Jonsson KB, Carn G et al. The autosomal dominant hypophosphatemic rickets (ADHR) gene is a secreted polypeptide overexpressed by tumors that cause phosphate wasting. J Clin Endocrinol Metab 2001;86:497-500.
Segawa H, Yamanaka S, Ohno Y et al. Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in KLOTHO mice. Am J Physiol Renal Physiol 2007;292:F769-F779.
NHERF
Mahon MJ, Donwitz M, Yun CC et al. Na/H exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signalling. Nature 2002;417:858-61.
Shenolikar S, Voltz JW, Minkoff CM et al. Targeted disruption of the mouse NHERF1 gene promotes internalization of proximal tubule sodium-phosphate cotransporter type IIa and renal phosphate wasting. Proc Natl Acad Sci USA 2002;99:11470-5.
Hernando N, Déliot N, Gisler SM et al. PDZ-domain interactions and apical expression of type IIa Na/Pi cotransporters. Proc Natl Acad Sci USA 2002;99:11957-62.
PHEX
The HYP Consortium. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet 1995;11:130-6.
Boileau G, Tehehhouse HS, Desgroseillers L et al. Chraracterization of PHEX endopeptidase cathalitic activity: identification of parathyroid-hormone –related peptide 107-139 as a substrate and osteocalcin Ppi and phosphate as inhibitors. Biochen J 2001;355:707-13.
Bowe AE, Finnegan R, Jan de Beur SM et al. FGF23 inhibits renal tubular phosphate transport and is PHEX substrate. Biochem Biophys Res Commun 2001;284:977-81.
FGF23
The ADHR Consortium. Autosomal dominant hypophosphatemic rickets is associated with mutations in FGF23. Nat Genet 2000;26:345-8.
Shimada T, Mizutani S, Muto T et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 2001;98:6500-5.
Strewler GJ. FGF23, hypophosphatemia, and rickets:has phosphatonin been found? Proc Natl Acad Sci USA 2001;98:5945-6.
White KE, Carn G, Lorenz-Depiereux B et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilizes FGF-23. Kidney Int 2001;60:2079-86.
Shimada T, Muto T, Urakawa I et al. Mutant FGF23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endorinology 2002;143:3179-82.
Shimada T, Hasegawa H, Yamazzaki Y et al. FGF23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 2004;19:429-35.
Shimada T, Urakawa I, Yamazaki Y et al. FGF23 transgenic mice demonstrated hypophosphatemic richets with reduced expression of sodium phosphate cotransporter type Iia. Biochem Biophys Res Commun 2004;314:409-14.
Oncogenic Rickets
Brendt T, Craig TA, Bowe AE et al. Secreted frizzled-related protein 4 is a potent tumor derived phosphaturic agent. J Clin Invest 2003;112:785-94.
Kurose K, Sakaguchi N, Nasu Y et al. Decreased expression of REIC/Dkk-3 in human renal clear cells carcinoma. J urol 2004;171:1314-8.
Nozaki I, Tsuji T, Iijima O et al. Reduce expression of REIC/Dkk-3 gene in non-small cell lung cancer. Int J Oncol 2001;19:117-21.
Tsuji T, Nozaki I, Miyazaki M et al. Antiproliferative activity of REIC/Dkk-3 and its significant down-regulation in non-small cell lung carcinomas. Biochem Biophys Res Commun 2001;289:257-63.
Kobayashi K, Ouchida M, Tsuji T et al. Reduced expression of the REIC/Dkk-3 gene by promoter-hypermethylation in human tumor cells. Gene 2002;282:151-8.
Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signalling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol 2005;16:2373-84.
Tumoral calcinosis
Inclan A, leon P, Camjeo MG. Tumoral calcinosis. JAMA 1943;121:490-5.
Mitnick PD, Goldfarb S, Slatopolsky E et al. Calcium and phosphate metabolism in tumoral calcinosis. Ann Intern Med 1980;92:482-7.
Topaz O, Shurman DL, Bergman R et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet 2004;36:579-81.
Larsson T, Yu X, Davis SI et al. A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metab 2005;90:2424-27.
Larsson T, Davis SI, Garringer HJ et al. FGF23 mutants causing familail tumor calcinosis are differentially processed. Endocrinology 2005;146:3883-91.
FGF References
Saunders JW Jr. The proximo-distal sequence of the origin of the parts of the chick wing and the role of ectoderm. J Exp Zool 1948;108:363-403.
Naski MC, Colvin JS, Coffin JD et al. Repression of hedgehog signaling and BMP4 expression in growth plate cartilage by fibroblast growth factor receptor 3. Development 1998;125:4977-88.
Hu MC, Qiu WR, Wang YP et al. FGF-18, a novel member of fibroblast growth factor family, stimulates hepatic and intestinal proliferation. Mol Cell Biol 1998;18:6063-6074
Niswander I, Tickle C, Vogel A et al. FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell 1993;75:579-587.
Fallon J et al. FGF-2: Apical ectodermal ridge growth signal for chick limb development. Science 1994;264:104-7.
Sun X et al. Conditional inactivation of FGF4 reveals complexity of signalling during limb bud development. Nature Genet 2000;25:83-6.
FGF-Receptor linked diseases
Keegan K, Johnson DE, Williams LT et al. Isolation of an additional member of the fibroblast growth factor receptor family, FGFR-3. Proc Natl Acad Sci USA 1991;88:1095-9.
Rousseau F, Bonaventure J, Legeai-Mallet L et al. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature 1994;371:252-4
Delezoide AL, Benoist-lasselin C, Legeai-Mallet L et al. Spatio-temporal expression of FGFR 1,2 and 3 genes during human embryo-fetal ossification. Mech Dev 1998; 77:19-30.
Tavormina PL, Shiang R, Thompson LM et al. Thanatophoric dysplasia /(types I and II) caused by distict mutations in fibroblast growth factor receptor 3. Nat Genet 1995;9:321-8
Rousseau F, Saugier P, Le Merrer M et al. Stop codon FGFR3 mutations in thanatophoric dysplasia type I. Nat Genet 1995;10:11-12
Hecht JT, Herrera CA, Greebhaw GA et al. Confirmatory linkage of hypochondroplasia to chromosome arm 4p (letter). Am J Med Genet. 1995;57:505-6.
Prinstern C, Carrera P, Mora S et al. The two recurrent mutations of FGFR3cause hypochondroplasia in 57% of the Italian patients. Horm Res 1996;46:83 (Abstract)
Prinstern C, Carrera P, Del Maschio M et al. Comparison of clinical-radiological and molecular findings in hypochondroplasia. Am J Med Genet 1998;75:109-112.
Tavormina PL, Bellus GA, Webster MK et al. A novel skeletal dysplasia with developmental delay and acanthosis nigricans is caused by a Lys650Met mutation in the fibroblast growth factor receptor 3 gene. Am J Hum Genet 1999;64:722-31.
Colvin JS, Bohne BA, Harding GW et al. Skeletal overgrowth and deafness in mice lacking firoblast growth factor receptor 3. Nat Genet 1996;12:390-7.
Wang Y, Spatz MK, Kannan K et al. A mouse model for achondroplasia produced by targeting fibroblast growth factor receptor 3. Proc Natl Acad Sci USA 1999;96:4455-60.
Chen L, Adar R, Yang X et al. Gly369Cys mutation in mouse FGF-R3 causes achondroplasia by affecting both chondrogenesis and osteogenesis. J Clin Invest 1999;104:1517-25.
Su WC, Kitagawa M, Xue N et al Activation of STAT1 by mutant fibroblast growth-factor receptor in thanatophoric dysplasia type II dwarfism. Nature;1997:386:288-92.