TNF family growth factors
. Lymphotoxin alfa/TNF alfa
. lymphotoxin beta/TNF beta
. FAS Ligand
. Nerve Growth Factor
. CD40 ligand or CD154
. CD27 ligand
. CD30 ligand
. OX-40 ligand
. 4-IBB ligand
. RANK/ODF/SOFA (Receptor Activator of NF-kB/ Osteoclasts differentiation Factor)
. RANK Ligand
. OPG (Osteoprotegerin)
Receptors for TNFalfa
TNFR1: p55 - CD120a Tumor necrosis factor receptor I (Chr. 12)
TNFR2: p75 - CD120b Tumor necrosis factor receptor II
CD95 FAS
TNFRSF4: OX40, OX40 antigen
TNFRSF5: CD40 B cell associated molecule
TNFRSF9: 4-IBB homologue of mouse 4-IBB
TNFRSF11A: RANK, Receptor Activator of Nuclear factor kB (Chr.18q21-22)
TNFRSF11B: OPG, Osteoprotegerin (Chr. 8q24.2 )
TNFRSF16: Nerve Growth Factor Receptor
Recently a classification of these receptors have been reported all containing
1. FADD domain: a protein sequence thought to be Fas-Associated Death Domain: a bipartite bridge that directy binds to CD95 Ligand and to pro-caspase 8.
2. TRADD: an adapter protein with liker function with FADD sequence
The molecular structure of this receptor has been defined as formed by a 55 kDa protein with:
1. four cysteine –rich extracellular domains (the first two are involved in most mutations founded in syndromic complex)
2. Intracellular FADD domain involved in signal transduction through protein-protein interaction.
The receptors at present know are:
- TNF-R1: the true receptor for TNF alpha
- TRAIL-R1: using FADD sequense and linking to pro-caspase 8
- TRAIL-R2: using FADD sequence.
Soluble forms of these last two recptors are present in extracellular fluids, with an inhibitor action on apoptotic signals:
- TRAIL-R3 also know such as TRID
- TRAIL-R4 also know such as TRUNDD
We can consider OPG-Ligand and RANK-Ligand such as TRAIL like molecules ie TNF Related Apoptosis Inducing Ligands.
OPG (Osteoprotegerin) on the contrary is a TRAIL-Receptor soluble form ie with inhiting action on TRAIL-R induced apoptosis.
On the osteoclasts instead of linking to true TRAIL-Rs the ligands can link to RANK a specific receptor able to activate NF-kB.
Autoinflammatory diseases
The receptor activation of TNF alfa have been clearly demonstrated to be the masterplayers in regulation those we begin to call “autoinflammatory syndromes”.
These are represented by:
- Familial Mediterranean Fever - pyrin
- Familial Hibernian Fever – TNF receptor type 1
- Muckle-Wells syndrome - cryopyrin
- Familial cold urticaria - cryopyrin
- Chronic infantile neurological cutaneous and articular syndrome - cryopyrin
- Neonatal onset multisystem inflammatory disease - cryopyrin
- Hyperimmunoglobulinaemia D and periodic Fever syndrome – mevalonate kinase
First of all we have to remember that these syndromic complexes are very rare diseases and may be only a model of more large diffuse arthritic diseases. Anyway considering the genetic selectivity pressure involving the genes areas mutated in these subjects, we can suppose that the selectivity pressure has favored the selection of heterozygous people. People affected are from Mediterranean ancestral origin such as Sepharditic Jwes, Armenian, Turkish, Greek, Italian people and these diseases affect humans during early year of life. We can suppose that people heterozygous for such mutations react with a very intense systemic way leading to great survival chances.
The molecular pathway involves the cleavage of precursor of IL-1 beta before its secretion affecting a group of proteins showing the same molecular structure now called NALPs proteins able to activate the caspase-1 complex involved in degradation of pro-IL1 ( pyrin ) such in the case of Familial Mediterranean Fever.
The defect of mevalonate kinase involved into the genesis of hyperglobulinemia D is involved in an increased secretion of IL-1 beta that is linked to the production of isopreoid molecules, normally linking IL-1 to the plasmamembrane.
The defect of TNF receptor type 2 lead to an increase stimulation of TNF alfa, due to defect in cleavage site of this receptor normally acting such as scavenger receptor for circulating TNF alfa, such as the case of so called Familial Hibernian fever. The autosomal dominant inheritance of this syndrome has been reported in many ethnic groups but in particular in Irish and Scottish family. So far more than 20 families have been described in Australia, United States and Europe too. Linkage analysis mapped the susceptibility gene for two separate families to the short arm of chromosome 12. Identifying several missense mutations, at least 16, in the gene for the type 1 TNF receptor in the exons 2,3 and 4 of genomic sequence has lead to hypothesis that receptor activation usually lead to cleavage and shedding of its intracellular portion into the circulation, where it acts as an inhibitor of TNF alpha. Activation of receptor can leads to activation of a protease that shed the TNF receptor from cell surface. It is postulated that in affected patients the TNF receptor cannot be shed by proteases leading to persistent inflammatory response.
Familial meditterranean fever is defined such as disease linked to an alteration on TNFalfa Receptor but due to a pyrin defect. The prolonged attacks, conjunctivitis, and localized myalgias differentiated the TNF-receptor associated periodic fever from other syndromic periodic fever syndromes.
Finally cryopyrin defect is associated at different level with the last three syndromic complexes including neonatal-onset multisystem inflammatory diseases.
Cryopyrin belong to NALP superfamily, and is able to activate caspase system at N terminal site, to link to nucletide sequence in its central portion (Leucine rich repeat), and at C terminal site it shows many similaryties with Toll like receptor family able to link to many bacterial and host molecules. Finally a particular attention deserve the presence in these hereditary periodic fever syndromes of amyloidosis. It’s interesting to note that many bone diseases are characterized by the presence in abnormal level of amyloidotic structures in extracellular space. In particular it would be interesting to evaluate the different arhtropatyes affecting adults for the presence of amyloidotic proteins in synovial flluids or anyway in extracellular degraded matrix. In considerations of recent evidences of involvement of matrix metalloproteinases and aggrecanases gene mutations in diseases affecting cartilagineous structures, it would be intersting to evaluate the hypothesis of an increased production and secretion at kidney level of amyloid like proteins. The pathways of production of amyloid include the gamma secretase enzymes involvement into degradations of HLA linked moleculed in particular of beta2 microglobulin. It has been suggested that after the apototitc processes due to same mechanisms involving the increased secretion of IL-1 beta would produces increased amount of plasmamebrane linked proteins containing strucural motif able to form agregates of fibrils in extracellualr matrix that can only be secreted by kidney with possible damage of renal filtration at glomerular level. Interestingly in the disease affecting older people the levels of amyloid like structures are increased for example at level of cerebral tissue in degenerativve diseases such as Alzheimer’s diseases. The same mechanism would be present also at bone tissue livel in particular at articular level. It si interesting to note that amyloidosis is preset in Armenian people living in Armenia wheres it is absent in Armenian living elsewere; it is possible that the presence of only one allele of this gene responsible of autosomal recessive diseases, previously described, account for an increased production of amyloid proteins, production that require also a predisposition of SAA1 genotype in the amyloid precursors.
Arthritic diseases and malignancies
Virchow suggested in the nineteenth century that chronic inflammation might give rise to malignancy, and the link between inflammation and cancer was not widely inderstood until recently. Clinical evidences of this link is demonstrated by the relationships between chronic infections with hepatitis B virus (HBV) and hepatitis C virus with hepatocellular carcinoma; infections with Helicobacter pylori and association with most gastric cancers; chronic inflammatory bowel diseases, such as Ulceratice colitis, and colorectal cancer; chronic airway irritation and inflammation caused by airborne partocles and tobacco smoke and lung carcinomas. Thus epidemiological studies were an excellent source of new working hypothetis concerning the pathogenesis of cancer. Hanahan and Weinberg summarized the processes underlying the emergence of neoplasia with the presence of self sufficiency in growth signals, insensitivity to growth inhibition signals, evasion from apoptosis, limitless replicative potential, tissue invasion and sustained angiogenesis.
Osteitis Deformans or Paget’s Disease of bone
Paget’s disease of bone is also called Osteitis Deformans and it is a more common metabolic disorder resulting from rapid bone remodelling. Interestingly on 1% of affected patients an osteosarcoma, fibrosarcomas or chondrosarcomas are present ; the more common site sarcoma transformed is the femur; higher risk patients are those with polyostotic form of the disease, and it has been linked to the presence of a single gene mutation tightly located on chromosome 18. So that Osteitis Deformans can be considered a precancerous lesion of bone.
Cardiovascular system is affected in polyostotic form with an increase in cardiac output during the active resorbing phase; cardiac failure is rarely present. The increase vascular flow rate is the first sing of Paget disease of bone when it stops after a correct antiresorptive therapy.
The hematic flow increase on skull bone can lead to a cerebral ischaemic attacks like those we see during vertebrobasilar insufficiency.
Alkaline phoshatase linked diseases
Inherited diseases are known to be associated with an alteration on ALP secretion:
. Hypophosphatasia: low levels of NTS-ALP due to chromosomal alteration on chromosome 1.
. Aphosphatasia: heritable deficit of NTS-ALP
. Inherited Hyperphosphatasia (Paget disease of bone)
Aphosphatasia
Is an heritable deficit of NTS-ALP where we have a typical biochemical characterization due to increased levels of:
- Phosphoetanolamine
- Pyridoxate phosphate (Vit. B6)
- Pyrophosphates
All molecules targets of ALP activity under normal conditions.
Recently, after the discovery of Osteoprotegerin as a “uncoupling factor” able to modulate bone remodelling, many studies have been devoted to unravel the gene location and transcription control of Osteoprotegerin-coding genes.
At present two chromosomal loci have been identified coding for two different kind of OPG-like proteins:
1. TNFRSFIIA on chromosome 18q21-22
2. TNFRSFIIB on chromosome 8q24.2
These gene locations have been identified thanks to the study of rare inborn errors of bone metabolism by White’s MP group, and in particular of
- Familial Expansile Osteolysis – MIM174810 - TNFRSFIIA
- Expansile skeletal Hyperphosphatasias – MIM 239000 - TNFRSFIIB
- Early onset Paget’s disease of bone in Japan
- Idiopathic Hyperphosphatasia (Juvenile Paget’s disease) MIM 239000
OPG, a soluble member of the superfamily of TNF receptors, is normally secreted in vivo into the marrow space by cells derived from embrional mesenchimal tissue. Physiological actions of OPG is to function as a decoy receptor free into extracellular space, necessary and sufficient for osteoclasts development.
KO mice for TNFRSFIIB develop osteoporosis with numerous osteoclasts and rapid remodelling bone tissue.
OPG serum levels have been described to be undetectable in patients affected by Hereditary Hyperphosphatasia (Juvenile Paget’s disease) according to the model described above.
TNFRSF11B: Osteoprotegerin (Chr. 8q24.2 ) mutations and osteoporosis
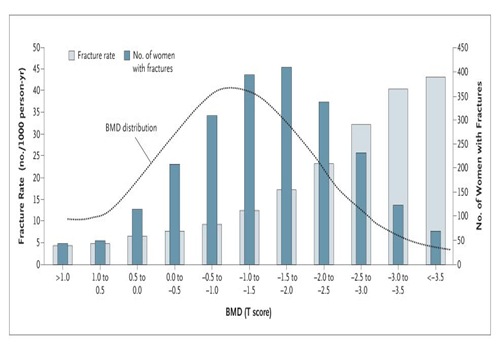
Interestingly, some recent data demonstrated the presence of a genetic polymorphism in the codon 3 at exon 1 of gene coding for OPG, causing an aminoacid substitution from lysine to asparagine at position 3. This gene mutation is presented in osteoporotic fractured older women. Several studies have reported an association between the Asn (C) allele presenting the mutation of Lys3Asn and increased lumbar spine BMD, as well’s lower risk of osteoporosis and osteopenia, with a reduced fracture risk.
Also the Study of Osteoporotic Fractures Research Group (SOF Research) studying 6695 women aged 65 years and older confirmed the previous studies demonstrating an association between high BMD level at diffeent skeletal sites and Ans3Asn (C/C) genotype. In particular C/C genotype is associated in SOF study women with high BMD values at calcaneal level, distal radious, intertrochanter, and lumbar spine. However it is important to note that also fracture risk was strictly related to OPG polymorphism. Interestingly, many of skeletal sites examined showed an association only with high BMD level but not with fracture risk, whereas the largest increase in fracture risk was seen at femural neck level, where there was no significant association with BMD values.
Studies evaluating serum concentration levels of OPG with BMD or fracture risk in humans have yielded conflicting results, partly because serum concentration levels of OPG, don’t reflect the OPG biologic activity within the bone microenvironment.
Genetically determined differences in OPG expression or function may be more reliable indicator of long term OPG activity. This possibility is supported by the association between the OPG Lys3Asn missense polymorphism and the risk of fracture over an average of 13.6 years of follow up in the SOF Research Group. Interestingly, the Lys3 Asn mutation occurs in a potential exonic splicing enhancer site. Changes in these sequences can have functional effect on the protein biologic activity. These findings for OPG Lys 3 Asn polymorphism are consistent with the common disease, common variant model inwhich high frequency alleles may contributes a modest relative risk but an appreciable proportion of disease burden in the population.
Aseptic losses of periprostetic bone
Inflammation reaction is strictly related to production and activation of membrane linked lipids mainly derivatives of arachidonic acid able to be transformed into their active metabolites: prostaglandins, leukotrienes, lipoxins and forming sfingolipids.
Between prostaglandins the main metabolic actions are exerted by PGE2 those receptors have been found in humans to be of 4 types EP1,2,3,and 4.
EP1 is linked to IP3 signaling and PLC with subsequent mobilization of intracellular calcium stores.
EP2 and EP4 stimulate formation of cAMP at intracellular level
EP3 activating Gi a G protein able to inhibits the enzyme adenyl cyclase.
Many data seems to demonstrate that the receptor responsible for induction of RANK-L expression by bone cells is represented by EP4.
PGE2 induction by RANKL seems to be mediated by EP4 receptor, so that double KO EP4 mice show few osteoblasts and showed a dramatic reduction in RANKL expression by osteoblastic cells.
Periprostetic osteolysis is a serious orthopedic problem often present at prosthesis / bone interface linked to activation of inflammatory prostaglandins: it is called “Aseptic Loosening of bone”. It leads to great limitation of many total joint replacement surgical interventions.
Wear debris production at tissue implant interfacce stimulates the activation of osteoclasts through RANKL secretion. Possible mediators of osteolytic action of wear debris are believed to be PGE2.
Interestingly sporadic reports of Bone Phenotype in “Darier Disease” suggested an involvment of SERCA 2b channels.
SERCA 2 b heterozygous mice -/+ showed a reduced frequency of calcium oscillation in bone cell membrane linked to loss of NFATc1 expression and loss of osteoclasts. SERCA 2b channels are present at cellular level at sarco-endoplasmic reticulum surface and they function such as Calcium ATPase type 2 channels involved in processes of osteoclasts differentiation.
These heterozygous mice showed a phenotype very similar to human bone phenotype of Darier disease showing bone tissue affected by bone cyst and fractures as well’s by periodontal gingival and mucosal inflammation.
References
Gafni J, Ravid M, Sohar E. The role of amyloidosis in familial Mediterranean Fever: a population study. Isr J Med Sci 1968;4:995-9.
Zemer D, Revach M, Pras M et al. A controlled trial of colchicine in preventing attacks of familial Mediterranean Fever. N Engl J Med 1974;291:932-4.
Dinarello CA, Wolff SM, Goldfinger SE et al. Colchicine therapy for familial Mediterranean Fever: double blind trial. N Engl J Med 1974;291:934-7.
Keat A. Reiter’s syndrome and reactive arthritis in perspective. N Engl J Med 1983;309:1606-15.
Goldenberg DL, Reed JI. Bacterial arthritis. N Engl J Med 1985;321:764-71.
Hoffmann G, Gibson KM, Brandt IK et al. Mevalonic aciduria – an inborn error of cholesterol and nonsterol isoprene biosynthesis. N Engl J Med 1986;314:1610-4.
Beutler B, Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med 1987;316:379-85.
Steere AC. Lyme disease. N Engl J Med 1989;321:586-96.
Cover TL, Aber RC. Yersinia enterocolitica. N Engl J Med 1989;321:16-24.
Bisno AL, Group A streptococcal infections and acute rheumatic fever. N Engl J Med 1991;325:783-93.
Pras F, Aksentijevich I, Gruberg I et al. Mapping of a gene causing familial Mediterranean fever to the short arm of chromosome 16. N Engl J Med 1992;326:1509-13.
Pinals RS. Polyarthritis and fever. N Engl J Med 1994;330:769-74.
Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med 1996;334:1717-25.
Case Records of the Massachussetts General Hospital (Case 25-1999). N Engl J Med 1999;341:593-9.
Drewe E, McDermott EM, Powell RJ. Treatment of nephrotic syndrome with etanercept in patients with the tumor necrosis factor receptor-associated periodic syndrome. N Engl J Med 2000;343:1044-5.
Drenth JP, van der Meer JWM. Hereditary periodic fever. N Engl J Med 2001;345:1748-57.
Hawkins PN, Lachmann HJ, McDermott MF. Interleukin-1 receptor antagonist in the Muckle-Wells syndrome. N Engl J Med 2003;248:2583-4.
Cundy T, Davidson J, Rutland MD et al. Recombinant osteoprotegerin for Juvenile Paget’s Disease. N Engl J Med 2005;353:918-23.
Deftos LJ. Treatment of Paget disease – Taming the wild osteoclast. N Engl J Med 2005;353:872-75.
Reid IR, Miller P, Lyles K et al. Comparison of a single infusion zolendronic acid with risendronate for Paget’s Disease. N Engl J Med 2005;353:898-908.
Kim K, Fisher MI, Xu SQ et al. Molecular determinants of esponse to TRAIL: in killing normal and cancer cells. Clin Cancer Res 2000;6:335-346.
Hugher EA, Ralston SH, Whyte MP et al. Mutations in TNFRSFIIA, affecting the signal peptide of RANK, cause Familial Expansile Osteolysis. Nat. Genet 2000;24:45-8.
White MP, Hughes AE. Expansile skeletal hyperphosphatasia is caused by a 15 base pair tandem duplication in TNFRSFIIA encoding RANK and is allelic to Familial Expansile Osteolysis. J Bone Min Res 2002;17:26-9.
White MP, Obrecht SE, Finnegan PM et al. Osteoprotegerin deficiency and Juvenile Paget’s disease. N Engl J Med 2002;347:175-84.
Langdahl BL, Carstens M, Stenjaer L et al. Polymorphisms in the osteoprotegerin gene are associated with osteoporotic fractures. J Bone Miner Res 2002;17:1245-55.
Arko B, Prezelj J, Kocijancic A et al. Association of the osteoprotegerin gene polymorphisms with bone mineral density in postmenopausal women. Maturitas 2005;51:270-9.
Zhao HY, Liu JM, Ning G et al. The influence of Lys3Asn polymorphism in the osteoprotegerin gene on bone mineral density in Chinese postmenopausal women. Osteoporosis Int. 2005;16:1519-24.
Wynne F, Drummond F, O’Sullivan K et al. Investigation of the genetic influence of the OPG, VDR (Fok1), and COL1A1 Sp1 polymorphisms on BMD in the Irish population. Calcif Tissue Int 2002;71:26-35.
Vidal C, Brincat M, Xuereb Anastasi A. TNFRSF11B gene variants and bone mineral density in postmenopausal women in Malta. Maturitas 2006;53:386-95.
Moffett SP, Oakley JI, Cauley JA et al. Osteoprotegerin Lys3Asn polymorphism and the risk of fracture in older women. J Clin Endocrinol Metab 2008;93:2002-8.
Samelson EJ, Broe KE, Demisse S et al. Increased plasma protegerin concentrations are associated with indices og bone strength of the hip. I Clin Endocrinol Metabol 2008;93:1789-95.
Darier disease and periprostetic aseptic bone losses:
Kariya Y, Homma M, Aoki S et al. Vps33a mediates RANKL storage in secretory lysosomes in osteoblastic cells. J Bone Miner Res 2009;24:1741-52.
Tsutsumi R, Xie C, Wei X et al. PGE2 signaling through the EP4 Receptor on fibroblasts upregulates RANKL and stimulates osteolysis. J Bone Miner Res 2009;24:1753-62.
Yang Y-M,Kim MS, Son A et al. Alteration of RANK-L induced osteoclastogenesis in primary cultured osteoclasts from SRCA2-/+ mice. J Bone Mineral Res 2009;24:1763-9.
Frezzini C, Cedro M, Leao JC et al. Darier disease affecting the gingival and oral mucosal surfaces. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102:e29-e33.
Menne T, Nielsen AO. Bone cysts and spontaneous fractures in two siblings with dyskeratosis follicularis Darier. Acta Derm Venereol 1978;58:366-7.
Ahn W, Lee MG, Kim KH et al. Multiple effects of SERCA2b mutations associated with Darier’s disease, J Biol Chem 2003;278:20795-801.
Sheridan AT, Hollowood K, Sakuntabhai A et al. Expression of sarco/endoplasmic reticulum Ca2 ATP ase type 2 isoforms (SERCA2) in normal human skin and mucosa , and in Darier’s disease skin. Br J Dermatol 2002;147:670-4.